… What is the origin of the dew ?
Dew as a physical phenomenon
“Dew is made of water. It forms during the night, when the sky is clear”. Everything is summarized in these few words.
Dew is the nearly magical result of the transformation of water vapor. This vapor, contained in our atmosphere, metamorphoses itself on a cold support into droplets of liquid water. The support can be colder than the ambient atmosphere because it yields its heat to the interstellar space by diffusing its light, the infra-red waves. The disordered thermal agitation of the vapor molecules in the air is transformed into liquid order thanks to the lower temperature and to the organized structure of the substrate: it is the phenomenon of condensation. The temperature at which supersaturated water vapor is transformed into liquid water, is called the “dew temperature”.
Drops of rain and fog are born, also, on substrates, but on microscopic substrates: dust, sand grains from the desert, spray of sea water… which are the various germs favoring condensation. We can say that rain and fog are the dew of the sky.
The importance of the radiative exchanges in the temperature of the surfaces where dew forms was suspected to contribute very early in the formation of dew ; this underlying hypothesis is found in the first book on this subject, by William Charles Wells “An essay on dew, and several appearances connected with it”, published in 1814 in London.
The radiative exchanges have, to simplify things, two antagonist actions: heating, by solar radiation, and cooling, mainly by infra-red emission, that is, the radiation emitted by a material at ambient temperature. During day, direct or indirect heating by the sun is greater then radiative cooling. During night, without solar heating, the substrate cools. Obviously, the greenhouse gases present in the atmosphere, like carbon dioxide and especially water vapor, limit the infra-red cooling. Cooling can even be canceled out when the cloud cover is dense : it is for this reason that dew forms only during the clears nights.
If dew is appreciated the freshness it brings to the early summer morning, and for the moisture it gives to the plants, even in dry periods, whilst white frost is less appreciated when it appears, generally in October, and makes us scrape the windshields of our cars before going to work.
If white frost exists, this is because the temperature of the surface goes down below zero degree Celsius and that the dew droplets have frozen. These cold droplets continue to grow bigger in the shape of dew needles of ice which form the white frost, a true “crystal” of dew. Dew and white frost however both arise in the same manner and require the same conditions: a clear night sky, strong radiation, then a fall temperature, a quiet wind and strong air moisture.
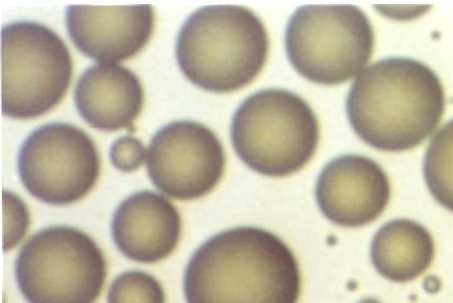
The dew droplets grow according to simple rules, that lead to an astonishing order. When small droplets are born on a surface, they are of very small size, of order of some millionth of millimeter. They grow bigger by agglomerating the vapor molecules around them: drops are real pumps of molecules. While increasing their size, they touch and fuse together – it is the phenomenon of coalescence – and form a new drop, larger and of the same form, but occupying less place than the two preceding drops. The result is surprising, but it is simply due to the fact that the drops “grow” in a dimension different from that of the surface, the third dimension. Whatever the sizes of the droplets, whether they are very small, of dimensions comparable to the atomic lengths used during the elaboration of thin layers for use in nano-electronics, or a million times larger such as the drops which we observe with the naked eye on leaves in the garden or the kitchen window pane, almost half of the surface will remain dry permanently. This results in “universal” properties for dew, which we will not detail here, but which makes this model very popular among the scientists who study the statistical properties of matter. And one can even make “jump” the drops of dew ! If the temperature of the solid on which dew grows is close to its melting point, the (latent) heat that the solid must evacuate so that the disordered vapor molecules “calm down” and organize themselves in liquid, is sufficient to liquefy the substrate. The drop truly “jumps” the dance of Saint-Gui.
The dewdrops can even “speak”: when they touch and fusion, they emit a small “cry” which can be made audible.
https://www.encyclopedie-environnement.org/en/air-en/dew/
